The three main families of PE films
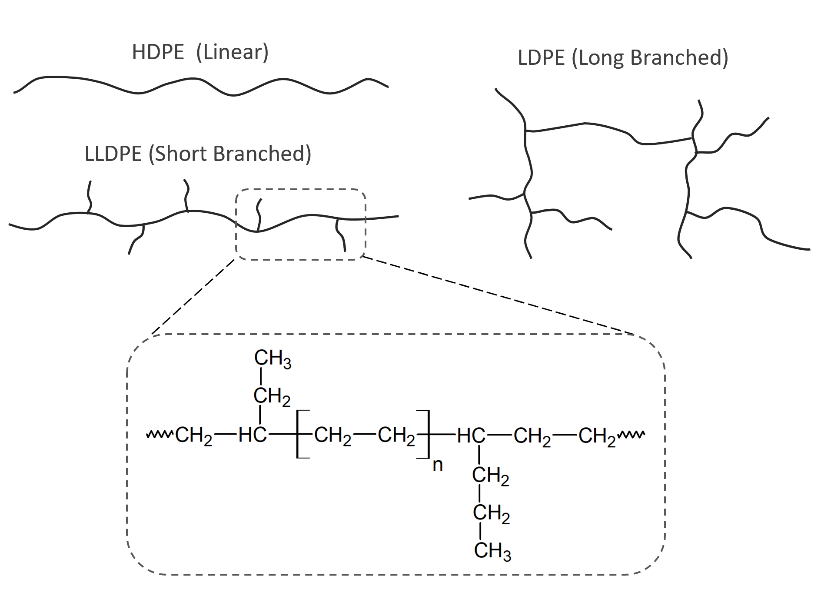
Polyethylene (PE) is one of the most common films used in today’s market. There are three main families of the PE films, which are LDPE (low-density PE), LLDPE (linear low-density PE), and HDPE (high-density PE). LDPE is best in clarity, most resistant to tear, and as the lowest stiffness. LLDPE has higher stiffness when compared to LDPE and is somewhat resistant to tear and has the greatest potential for downgauging. HDPE has the highest stiffness, but it is less resistant to tear and has the highest tensile strength. HDPE is the best barrier material of the three.
When evaluating the properties of the film, we can approach it from the three aspects of the film’s molecular structure. 1. Average molecular weight 2. Molecular weight distribution 3. Crystallinity or density.
|
|
LDPE |
LLDPE |
HDPE |
|
Average Molecular weight (g / mol−1) |
8.9 × 104 to 4.7 × 105 |
5.0 × 104 to 5.0 × 105 |
103–107 |
|
Molecular weight distribution (dispersion) |
30 |
<< 30 |
6 to 12 |
|
Crystallinity or density (g/cm3) |
35 to 55% (0.916 to 0.925) |
35 to 60% (0.9 to 0.939) |
up to 85% (0.941 to 0.96) |
Average Molecular weight – Atoms of different elements have different atomic weights, for instance, the atomic weight of carbon is 12 and the atomic weight of hydrogen is 1. Every polyethylene consists of multiple atoms forming molecular chains. As the average molecular weight increases, resin toughness increases. The tensile strength and resistance to cracking will also increase.
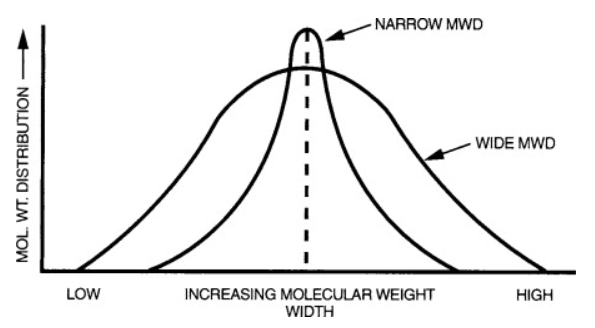
Molecular weight distribution – The relative distribution of molecular chains in a polyethylene resin is important to its properties. When there is a low variance in the chain length, the resin is said to have a narrow molecular weight distribution. On the other hand, when there is a high variance in the chain length, the resin is said to have a broad weight distribution. In general resin with narrow molecular distributions have greater impact strength and greater ease of processing.
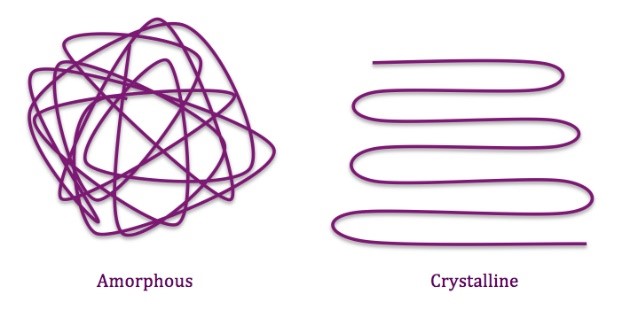
Crystallinity or density – Resins have a mixture of crystalline areas and amorphous areas. Molecular chains in crystalline areas are arranged parallel to each other. In the amorphous areas, chains are randomly arranged. The more molecular chains in the amorphous area will distribute a rubber-like property. The more molecular chains in the crystalline area will distribute a harder and brittle property. The higher the resin density, the higher degree of crystallinity.

Article by Daywey Chen, KYMC